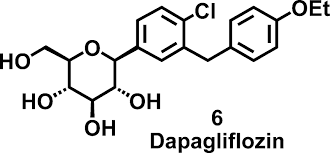
High-quality formulation with proven bioequivalence
? API and FDF integrated development
? EU GMP & EAEU GMP production support
? Fast-track registration support in CIS, MENA, Asia
Manufacturing Capabilities:
? Pilot to commercial scale batches
? Analytical method transfer & validation
? Full documentation and tech transfer package
Holder Pharm Services:
? Contract development & manufacturing
? Regulatory support & BE study coordination
? Supply chain management from China to your market


 2006-2025 上海博華國際展覽有限公司版權(quán)所有(保留一切權(quán)利)
滬ICP備05034851號-57
2006-2025 上海博華國際展覽有限公司版權(quán)所有(保留一切權(quán)利)
滬ICP備05034851號-57